Contact
Email: skarade@umd.edu
Call: (240) 314-6126
Sharanbasappa Karade (he/him)
Assistant Research Scientist
Mariuzza Group
Contact
Email: skarade@umd.edu
Call: (240) 314-6126
Education
Assitant Research Scientist (June 2023- current)
Post-Doctoral Associate (June 2018-May2023)
Ph.D. in Structural biology, (Jan 2012-Dec2017)
Profile
Sharanbasappa Karade received his M.Sc. in Bio-technology (2010) from RTM Nagpur University and Ph.D. in structural biology (2017) from JNU, Delhi, India. Currently working as, Assistant Research Scientist at IBBR-University of Maryland (since 2018). The area of research is Medicinal Structural biology.
ERQC machinery as broad spectrum Antivirals
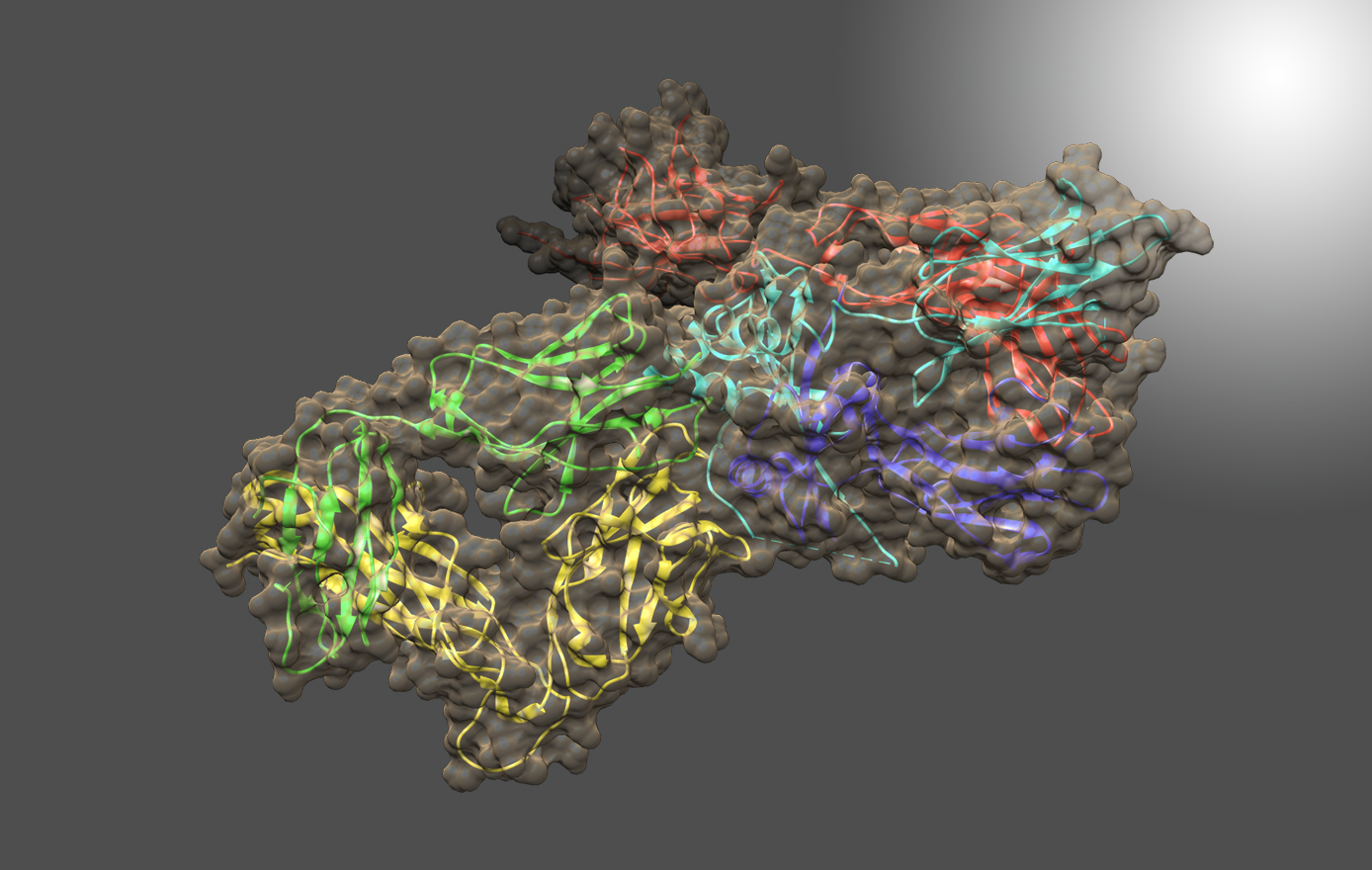
All viruses depend on host cell proteins for replication. Denying viruses’ access to the function of critical host proteins can result in antiviral activity against multiple virus families. In particular, small-molecule drug candidates which inhibit the α-glucosidase enzymes of the endoplasmic reticulum (ER) translation quality control (QC) pathway [a-GluI] have demonstrated broad-spectrum antiviral activities and low risk

for development of viral resistance. Crystal structures of Ct α-GluI in complex with UV-4, UV-5, and EB-0159 revealed extensive interactions with all four enzyme subsites and provided insights into the catalytic mechanism. Identification of ER Ct α-GluI as a surrogate for mammalian α-GluI will accelerate the structure-guided discovery of broad-spectrum antivirals. The study also highlights Ct as a source of thermostable eukaryotic proteins, such as ER α-Glu I, that lack orthologs in bacterial or archaeal thermophiles.