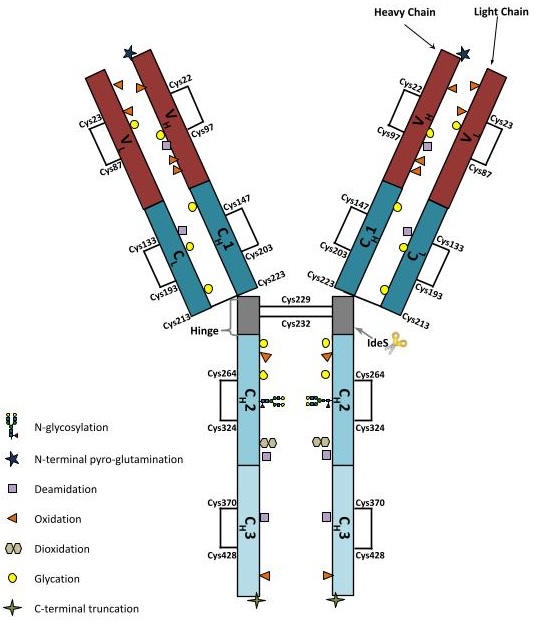
NISTmAb Overview
A vial of RM 8671 contains 800 µL of 10 mg/mL IgG1κ monoclonal antibody in 12.5 mmol/L L-histidine, 12.5 mmol/L L-histidine HCl (pH 6.0). The material has undergone industry standard upstream and downstream purification to remove process related impurities.
NISTmAb Technical Definitions
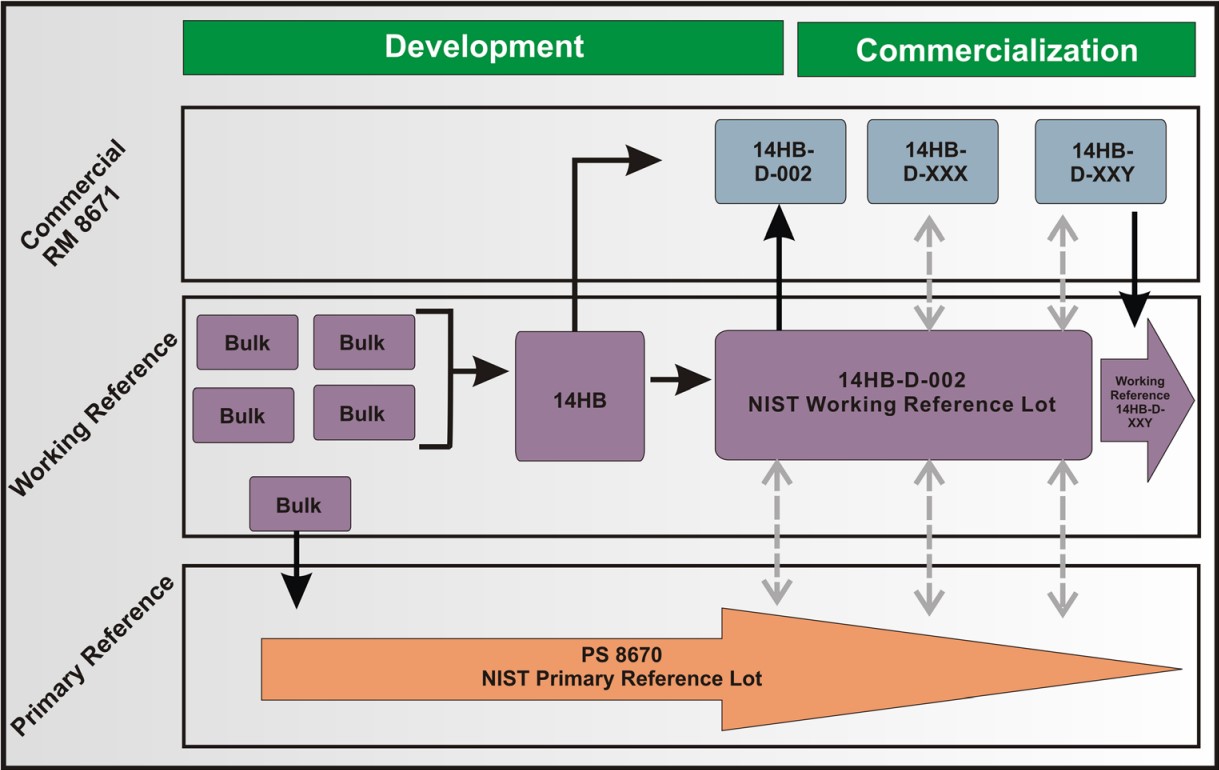
Reference Material 8671 (RM 8671) NISTmAb derived from multiple production lots (using the same process as PS 8670) that was homogenized and diluted as the publicly available reference material. Publicly available at 10 mg/mL in 800 uL aliquots.
Primary Sample 8670 (PS 8670) NISTmAb derived from a single production lot early in the product lifecycle and used during the initial development stage. This lot was reserved as the final in-house primary reference standard. Material is reserved for NIST in-house use and interlaboratory studies as needed (10 mg/mL and 100 mg/mL)
Reference Material Information Sheet (RMIS) Official NIST document describing material properties, NIST assigned values, intended use, and proper handling instructions.
Lifecycle management plan: NISTmAb material handling plan devised to follow industry standard practices and provide long-term availability of material with consistent product quality attributes. A brief figure summarizing this plan is below, while a detailed discussion can be found here .