NISTmAb Program
 The NIST monoclonal antibody (NISTmAb) reference material, RM 8671, is intended for use in evaluating the performance of methods for determining physicochemical and biophysical attributes of monoclonal antibodies. It also provides a representative test molecule for development of novel technology for therapeutic protein characterization. The RM is intended for a variety of uses that may include system suitability tests, establishing method or instrument performance and variability, comparing changing analytical test methods, and assisting in method qualification.
The NIST monoclonal antibody (NISTmAb) reference material, RM 8671, is intended for use in evaluating the performance of methods for determining physicochemical and biophysical attributes of monoclonal antibodies. It also provides a representative test molecule for development of novel technology for therapeutic protein characterization. The RM is intended for a variety of uses that may include system suitability tests, establishing method or instrument performance and variability, comparing changing analytical test methods, and assisting in method qualification.
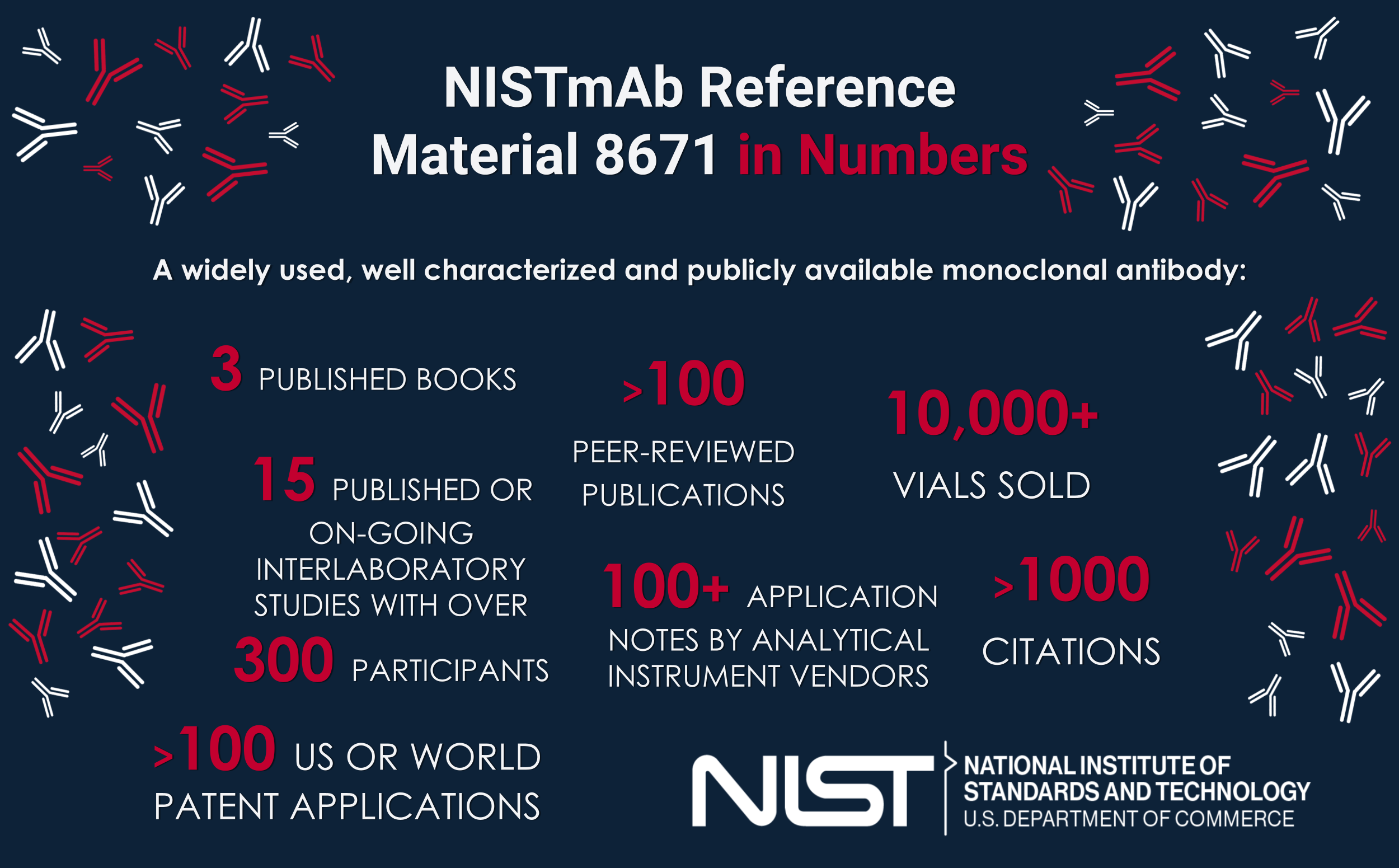
NISTmAb is, quite possibly, the most widely characterized publicly available monoclonal antibody, a molecule directly relevant to the biopharmaceutical industry. It is grounded-sm in quality measurements, thus providing a common control material for originator and follow on manufacturers alike. The voluntary and open access nature of this material makes it the premier choice for technology development in the pre-competitive space.
NISTmAb - Open Innovation
Comprehensive analysis of monoclonal antibody therapeutics is no easy
task. These molecules embody various complex attributes, the
characterization of which is a long and arduous process, yet monoclonal
antibody therapeutics have taken residence as perhaps one of the most
influential therapeutic classes of our time. An inter-continental
crowdsourcing characterization of a single IgG1κ (NISTmAb) was
reported as a three volume book series, serving as a supportive tool in the
evolution of analytical and biophysical methodologies. The NISTmAb
case study provides a comprehensive overview of monoclonal antibody
therapeutics, using the NISTmAb as a vehicle for highlighting the
characterization stages of product development. Contributors utilized the
NISTmAb throughout, demonstrated the potential utility of class-specific
reference materials as a means to facilitate open innovation, and identified
a number of emerging research areas for future development. Conclusion
of the series was therefore met with eager anticipation of continued
biopharmaceutical advancement through industry-focused partnerships.
Qualification, certification, and lifecycle management of the NISTmAb
reference material 8671, publicly released in 2016, was a representative
means by which this collaboration continued. In preparation of the
material for public availability, many methods were qualified for their
intended use in assessing the identity (e.g., peptide mapping), purity (e.g.,
capillary zone electrophoresis [CZE]), monomeric purity (size exclusion
chromatography [SEC] and capillary sodium dodecyl sulfate
electrophoresis [CE-SDS]), and stability (dependent on attributes) of the
NISTmAb. Additional characterization assays of dynamic light scattering
and flow imaging analysis of protein particulates were also employed.
Forced degradation studies were performed in order to further elucidate
potential degradation pathways and production of product-related
impurities relevant for challenging methods during qualification exercises.
Accelerated stability studies were also performed to identify adequate
storage and handling criteria appropriate to the materials intended use.
Development of innovative technology at NIST and in collaboration with industry stakeholders has also continued. Three interlaboratory studies have been completed using the NISTmab to evaluate the precision of hydrogen deuterium exchange mass spectrometry, nuclear magnetic resonance spectrometry, and glycoanalysis. The NISTmAb is also serving as the current basis for advancing measurement techniques at NIST such as small angle neutron scattering, nuclear magnetic resonance, x-ray diffraction crystallography, small angle X-ray scattering, mass spectrometry multi-attribute method, and glycan and peptide mass spectral libraries, to name a few.
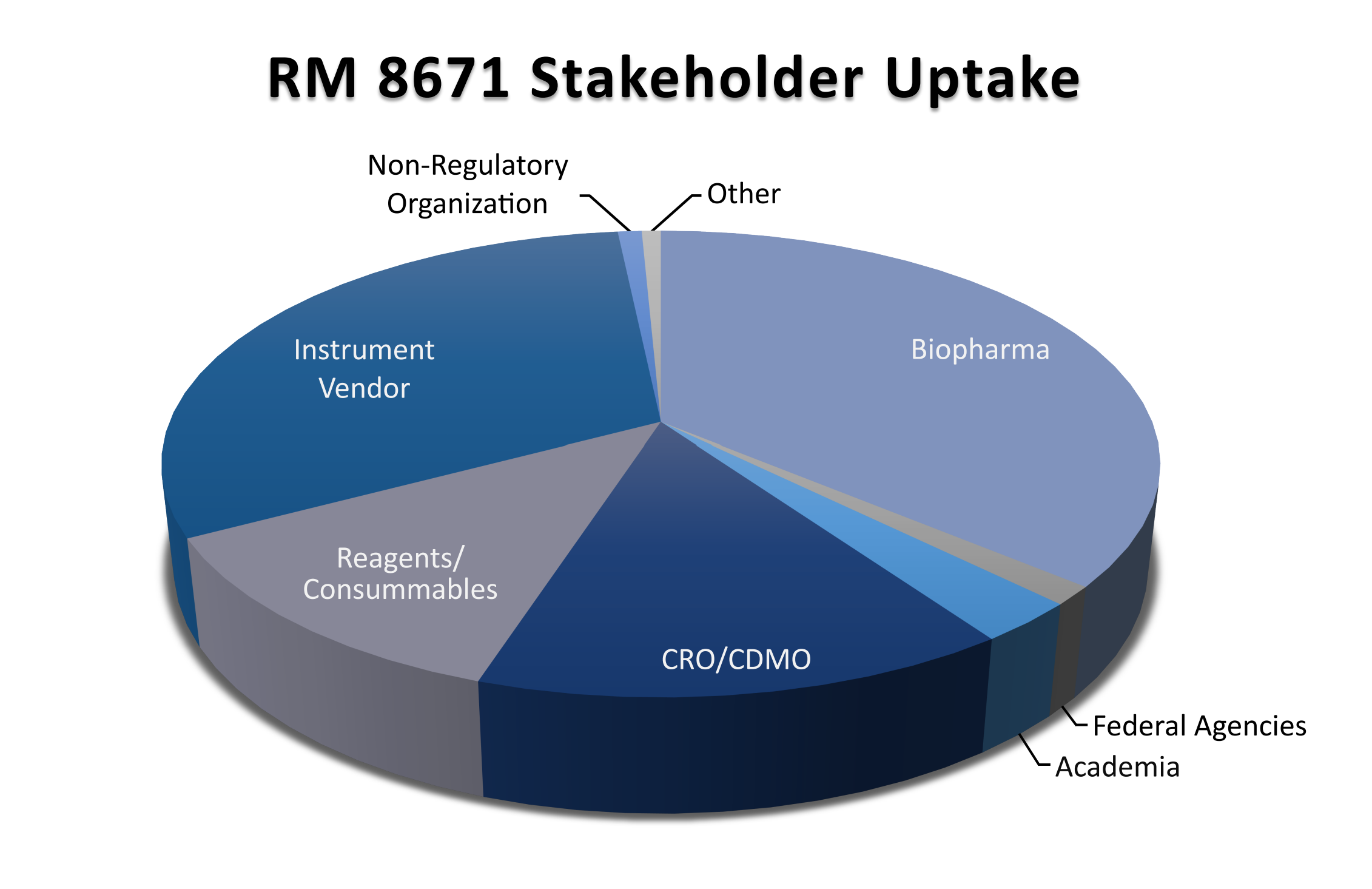
We hope this material finds widespread utility in the biomanufacturing community. We look forward to industry feedback on the technical utility of NISTmAb RM 8671 as well as the suitability of related follow-on materials that may supplement this robust and critical class of therapeutic.