Peptides
Links
Biophysical Methods to Investigate the Interplay of Host Defense Mechanisms at Biological Membranes
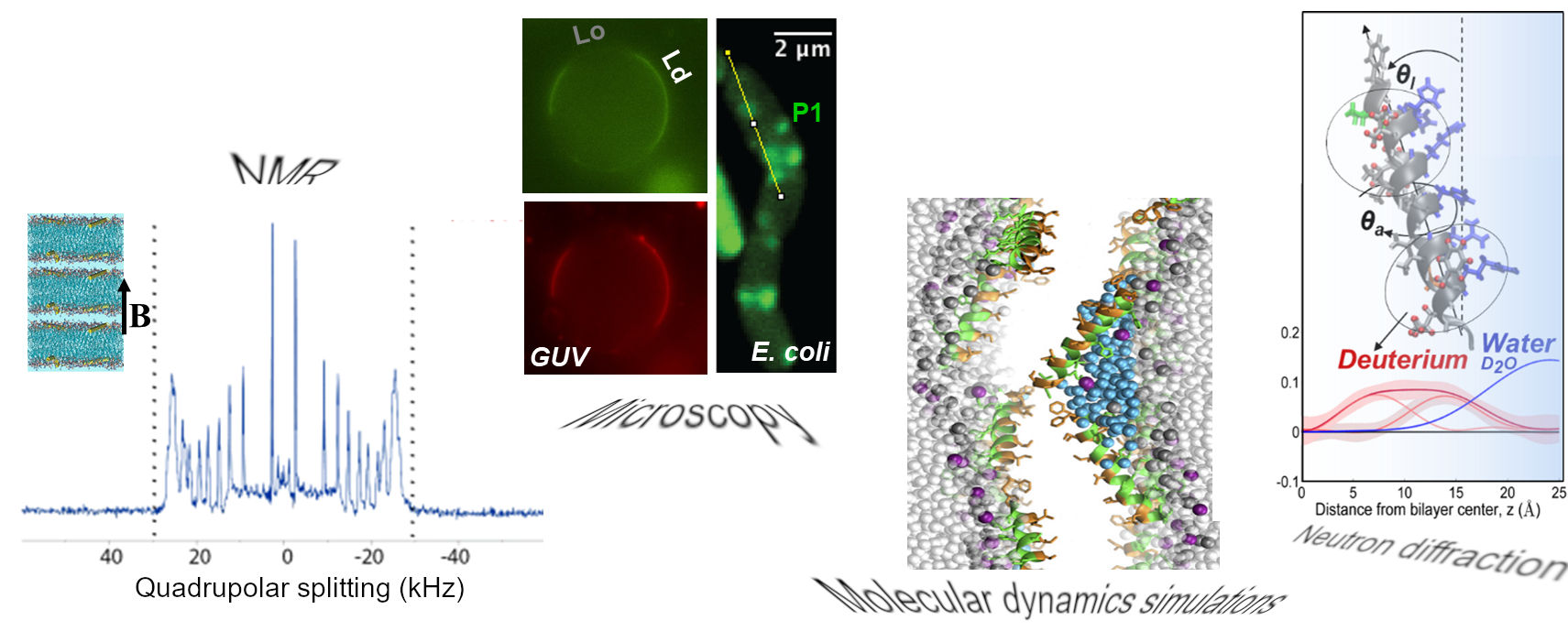
Overview
The innate immune response, which is the first line of defense against an infection or injury, is mediated by a variety of immune effector molecules that regulate molecular recognition at the plasma membrane. During the first phase of the response, pathogens are targeted by constitutively produced proteins (e.g. host-defense peptides (HDPs)) that feature evolution-tested efficacy against a broad range of pathogenic cells, including bacteria, viruses, and fungi. While they differ greatly in sequence, length (6-50 residues) and susceptibility to changes in pH and ionic strength, they share a series of physicochemical features (e.g. amphipathic secondary structures, rich in cationic residues).
Several naturally-occurring HDPs have an amino terminal copper and nickel (ATCUN) binding motif. Some of the host defense metallo-peptides form a complex with Cu(II) that leads to lipid peroxidation and formation of reactive oxygen species (ROS), DNA damage, and enhanced biological activity. This suggests that ATCUN-HDPs have conformations and dynamics that allow them to get metallated in the surrounding environment and adopt membrane positions that are conducive to structural and chemical disruption of pathogenic membranes, and thus bacterial death. While biological cell-based assays have been useful in demonstrating the antimicrobial importance of the ATCUN motif, they have not provided direct information to explain these effects on a molecular level. The role of the metal in the activity of these peptides at the membrane level is not well understood. On the one hand, ROS formation in the surrounding bilayer environment has structural and functional implications for membranes stability. On the other hand, binding of the metal ion can change peptide conformation and interactions with the lipids and water, leading to an increased ability of the peptides to perturb and permeate the bilayer.
In an integrated effort with research groups at College of William and Mary (Cotten lab), University of Connecticut (Angeles-Boza lab), and National Institutes of Health (Pastor lab) scientists at IBBR are exploring the structure-function relationship of HDPs at lipid membranes by employing surface-sensitive techniques, neutron scattering methods, solid-state NMR, MD simulations and in vivo assays. Knowledge learned from these studies will help us delineate principles of HDP mechanisms of action, host-pathogen interactions and the role of evolutionary-conserved structural motifs.
