-preview.jpg)
Contact
Email: tfuerst@umd.edu
Call: (240) 314-6507
Thomas Fuerst
Professor
Fuerst Group
Contact
Email: tfuerst@umd.edu
Call: (240) 314-6507
Education
- Ph.D., Molecular Genetics, Cornell University, 1984
- MBA, Science, Technology and Innovation, George Washington University, 1992
- B.A., Biochemistry, University of California at Berkeley, 1980
Profile
Dr. Fuerst’s research is focused on the development of next-generation vaccines and protein-based therapeutics for infectious disease and cancer. The Fuerst group brings together an assemblage of scientific disciplines including virology, immunology, analytical chemistry, cell biology, structural biology, computational biology, and protein engineering. The multidisciplinary programs include: (1) a structure-based vaccine design program focused on enveloped viruses, (2) a scaffold-based protein therapeutics program focused on cancer targets, and, (3) an immunoadjuvant and delivery program focused on polyphosphazene-based macromolecular delivery systems.
CURRENT RESEARCH

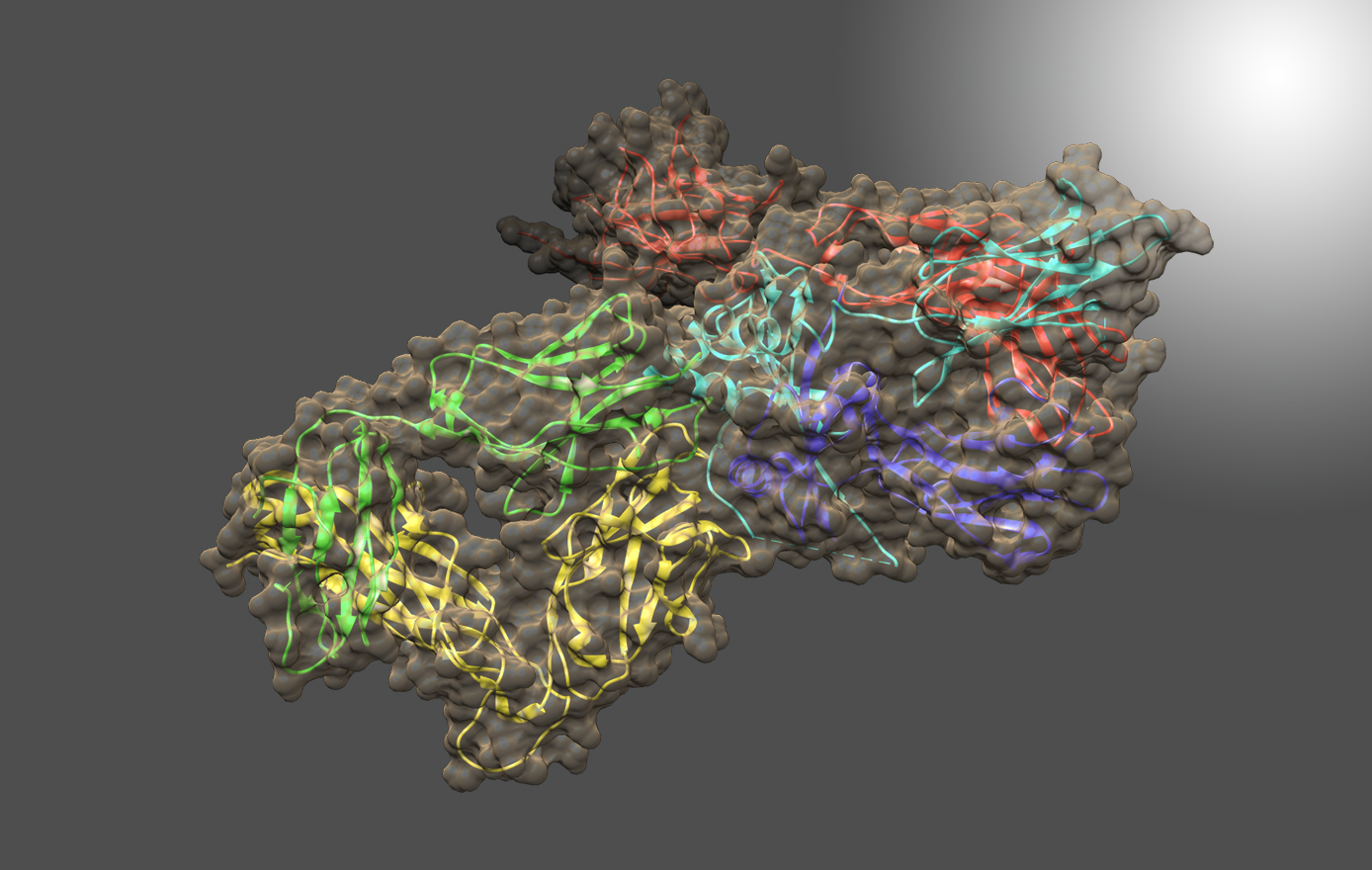
The structure-based vaccine program identifies viral proteins that can be used in vaccines to promote protective immune responses. Hepatitis C virus (HCV), a major human pathogen and a leading cause of liver cirrhosis, liver failure, and hepatocellular carcinoma, is the principal focus of this program. Multiple research studies have suggested that different arms of the immune response are needed for controlling acute and chronic HCV infection. The Fuerst group is defining conserved portions of HCV proteins that can promote protective antibody responses against multiple HCV strains. This novel approach relies on the fundamental principles of structural vaccinology, which involves understanding the nature of neutralizing determinants at the atomic level and applying these insights to develop vaccines that induce protective responses.

drug delivery technologies.
The scaffold-based therapeutic program is developing powerful new classes of protein-based molecules, referred to as SMART molecules, as multi-component protein machines with the potential to undergo changes in conformation in response to binding, which activates a targeted response. SMART molecules can act with low toxicity and have fewer off-target reactions. The group is developing the technology using HRAS, one of the most frequently mutated oncogenes associated with numerous cancers. The SMART molecules under development are expected to sense subtle differences between normal versus oncogenic states in HRAS and compute different therapeutic responses.
The immunoadjuvant and delivery program is customizing a platform for synthesizing multifunctional, biodegradable classes of polymers well-suited for protein stabilization, antigen presentation, and delivery of macromolecules. The research group uses a unique class of polymer called polyphosphazene, which has specialized structural characteristics including a biodegradable backbone. Polyphosphazenes can undergo self-assembly with vaccine antigens and protein therapeutics, and they have unique targeting capabilities, including environmentally-triggered controlled release. Projects within this program focus on immunoadjuvant properties of polyphosphazenes for vaccine delivery and targeted nanoparticle delivery for protein-based therapeutics.